valence electrons selenium|how do you find valence electrons : Manila There are two ways to find the number of valence electrons in Selenium (Se). The first is to use the Periodic Table to figure out how many electrons Selenium has in its valence shell.. 3 Purchase an eligible LG Refrigerator and eligible LG Compact Refrigerator in a single transaction on www.lg.com and receive an instant saving at the point of sale for the value of the LG Compact Refrigerator. Discounted amount to equal the amount paid for the eligible LG Compact Refrigerator (before taxes, shipping, or handling). Products must be .
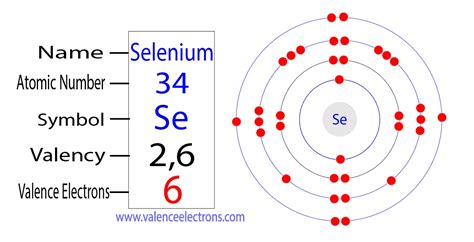
valence electrons selenium,There are two ways to find the number of valence electrons in Selenium (Se). The first is to use the Periodic Table to figure out how many electrons Selenium has in its valence shell..
This table of element valences includes the maximum valence and most .
March 23, 2023. Valence electrons: For main group elements (i.e s-block . In the chemistry branch of science, Selenium has recognition as the chemical element. It has the symbol of Se and the atomic number 34. Flerovium Valence Electrons. Helium Valence Electrons. . Selenium has 6 valence electrons because there are 6 electrons present in the outermost shell of the Selenium (Se) atom. Now let’s see how you can easily find .

Valence electrons are the outer-shell electrons of an atom. Valence electrons determine the reactivity of an atom. Atoms have a tendency to have eight .
Valence electrons are the outer-shell electrons of an atom. Valence electrons determine the reactivity of an atom. Atoms have a tendency to have eight .valence electrons selenium Valence electrons are the outer-shell electrons of an atom. Valence electrons determine the reactivity of an atom. Atoms have a tendency to have eight .
Selenium has 6 valence electrons. Methods. We can write the valence electrons of selenium using two different methods: #1 Using periodic table. #2 Using electron configuration. Let’s break down each .Electron configuration for selenium. The history of Selenium. Periodic table history. Identifiers. List of unique identifiers for Selenium in various chemical registry databases. . Valence electrons are the electrons which are located in the outermost shell of the atom or molecule. Selenium has 6 electrons in its outermost shell i.e. 2 electrons in s orbit and 4 electrons in the p .
Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
The atomic number of selenium is 34, which means it has 34 electrons. Now it is possible to find the orbital notation of selenium very easily through electron configuration. That is, the orbital notation of selenium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 4.
Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..
Selenium is a classified nonmetal and its symbol is ‘Se’. Selenium is the 34th element of the periodic table so its atomic number is 34. The atomic number of an element is equal to the number of protons and electrons in that element. Therefore, a selenium atom has thirty-four protons and thirty-four electrons.Answer and Explanation: 1. Become a Study.com member to unlock this answer! Create your account. View this answer. Valence electrons are electrons in the outermost energy level of an atom. Selenium is in the fourth period, so its valence electrons are in the. See full answer below.

Selenium is a member of the sulfur family with elements including tellurium and polonium. This family has six electrons in the outermost shell. Selenium specifically has an electron configuration of 2-8-18-6. The six electrons in the outermost shell allow selenium to have a variety of valence numbers. Selenium compounds have been found that .valence electrons selenium how do you find valence electrons Method 1: From the Periodic Table. To find out the valence electrons of Selenium, you have to see the position of selenium in the periodic table. More specifically, you have to see the group wise position of Selenium element in the periodic table. From the above image, you can see that the Selenium (Se) is present in the group 16 of periodic . sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group . Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable . As a gas or vapor, the halogens all had a pungent odor. After the development of quantum mechanics, it was shown that the halogens all had seven valence electrons, supporting their original placement into the same group on Mendeleev's periodic table. Figure 11.1.1 11.1. 1: Periodic table by Dmitri Mendeleev, 1871.
Selenium consists of 34 electrons distribution in its 4 orbits. So electronic configuration of selenium define as: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 3d10 4p 4. Or. The electronic configuration can also be .Orbital diagram. Selenium electron configuration. ← Electronic configurations of elements. Se (Selenium) is an element with position number 34 in the periodic table. Located in the IV period. Melting point: 217 ℃. Density: 4.82 g/cm 3 . Electronic configuration of the Selenium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 .Valence shell electrons (or, more simply, the valence electrons) are the electrons in the highest-numbered shell, or valence shell, while core electrons are the electrons in lower-numbered shells. We can see from .
A periodic table showing how many valence electrons the main groups have. Group 1 = 1 valence electron Group 2 = 2 valence electrons Group 13 = 3 valence electrons Group 14 = 4 valence electrons Group 15 = 5 valence electrons Group 16 = 6 valence electrons Group 17 = 7 valence electrons Group 18 = 8 valence electrons (except .
2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding its number of valence electrons is quite simple (except, of course, for the transition metals.) If you're given the configuration from the get-go, you can skip to the next step.
Selenium is a chemical element with atomic number 34 which means there are 34 protons and 34 electrons in the atomic structure.The chemical symbol for Selenium is Se. Electron Configuration and Oxidation States of Selenium. Electron configuration of Selenium is [Ar] 3d10 4s2 4p4. Possible oxidation states are +4,6/-2. Electron .
David Jin (UCD) Chemistry of Selenium (Z=34) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Element number 34, selenium, was discovered by Swedish chemist Jons Jacob Berzelius in 1817. Selenium is a non-metal and can be compared chemically to its other non-metal . Step-1: First, find the atomic number of selenium from periodic table. From periodic table ,we see that the atomic number of selenium is 34. Step-2: We know that the atomic number of selenium is 34.So selenium has 34 protons and 34 electrons as the charge of electrons and protons are equal but opposite in nature.The charge of proton .
valence electrons selenium|how do you find valence electrons
PH0 · valence electrons chart
PH1 · valence electrons calculator
PH2 · valence electron configuration calculator
PH3 · valence charge chart
PH4 · selenium valence electron configuration
PH5 · periodic table valence electrons
PH6 · list of valence electrons for each element
PH7 · how do you find valence electrons
PH8 · Iba pa